Unit of Rate Constant for Third Order Reaction
K 1 2t x 2a-x a 2a x2. For a reaction taking place at a boundary one would use moles of A or B per unit area instead The exponents m and n are.

Definition Of Rate Constant Chegg Com
Dimensional analysis requires the rate constant unit for a reaction whose overall order is x to be.

. The example and the different cases of their third order reaction are also. L3mol3sec mol2secL2 mol3secL63 Lmolsec L2mol2sec. Which of the following would be a reasonable unit for the rate constant of a third order reaction.
100 17 ratings for third order rate K A3 d. More generally speaking the units for the rate constant for a reaction of order m n m n are mol1mn Lmn1 s1. The unit of rate constant is the same as that of rate of reaction in Third order reaction Second Order reaction First order reaction Zero order reaction.
R kA B Where k is known as the rate constant if. In this reaction the rate will be written as. Is equal to three.
A reaction is said to be of third order if the rate is determined by the three terms of concentration variation. Here stands for the concentration or rather the order of the reaction with respect to the reactant. 3 The time taken to complete a half reaction half life period is inversely proportional to the square of initial concentration of the reactant.
If the reaction is zero-order the rate constant has exactly the same units as the rate of the reaction mol L 1 s 1If the reaction is first order then the concentration of one reactant takes care of the units of concentration thus the rate constant is simply given in units of reciprocal time s 1If the rate constant has the same unit as the rate of reaction the total order of. Rate Constant Units for Common Reaction Orders. Now let us derive the unit for the key for this type of reaction.
Rate k A 2 rate k A B Mt k M 2. M o l 2 o l 2. Unit of K litre2 mole -2 time-2.
The table below summarizes the rate constant units for common reaction orders. S -1 min -1 hr -1 etc. Solve any question of Chemical Kinetics with-.
The article defines their order reaction along with its derivation. Here kT is the reaction rate constant that depends on temperature and A and B are the molar concentrations of substances A and B in moles per unit volume of solution assuming the reaction is taking place throughout the volume of the solution. Rate k A Mt k M.
L2mol2 sec QUESTION 11 Given the data below for the reaction 2A 20-4CDE35 the reaction is order in A order in order in Cand order overall. Is the rate constant. Chemistry Objective type Questions and.
The units of the rate constant k depend on the overall reaction order. We review their content and use your feedback to keep the quality high. QUESTION 10 Which of the following would be a reasonable unit for the rate constant of a third order reaction.
View the full answer. So let us consider a third order reaction. 2 The unit of velocity constant depends upon the units of concentration because unit of K litre 2mole -2 time-2.
Overall Reaction Order x Rate Constant Unit L x1 mol 1x s 1 0 zero mol L 1 s 1. Although the specific units for concentration and time are indicated as molL and s any other valid units can be used. Rate Constants for Common Reaction Orders.
Units of rate constant for nth order mol lit 1 1nt 1For third order reaction n3 Units are mollit 1 13t 1mol l 2lit 2t 1. Mol 1 m n L m n 1 s 1. So I read law becomes rate.
The units of the rate constant of a third order reaction are. Is the concentration in Mueller. To put it another way the minimum number of molecules required for the reaction is three.
The units of k for a zero-order reaction are Ms the units of k for a first-order reaction are 1s and the units of k for a second-order reaction are 1Ms. Link summarizes the rate constant units for common reaction orders. The rate of the reaction 2NOO 2 2NO 2 is given by RatekNO 2 O 2 Since it is a third-order reaction the unit of the rate constant is mol-2 s-1.
The unit of the rate constant for a second-order reaction is Lmols or 1Ms and that for a third-order reaction is L 2 mol 2 s. In this video you will learn the Units of rate constant for zero order first order second order third order and nth order reactionUnits of rate constant. For a third order reaction the rate constant has units of liter squared per mole squares per second L 2 mol 2 s 1 or M 2 s 1 Other Calculations and Simulations For higher order reactions or for dynamic chemical reactions chemists apply a variety of molecular dynamics simulations using computer software.
Find the rate constant unit for the reaction 2NOO 2 2NO 2 1 Mark Ans. M o l 1 L 1 s 1. Rate Constant k has UNITS.
According to the Rate Law the rate of reaction is expressed by the concentration of reactants with moles raised to their powers and units of rate and rate constant will be the same for that order which does not depend on the concentration of reactants. And the small M.

Determining Rate Laws And The Order Of Reaction General Chemistry Jove
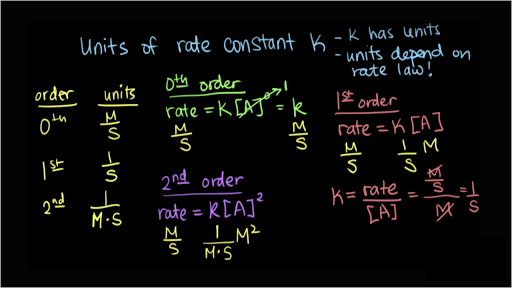
No comments for "Unit of Rate Constant for Third Order Reaction"
Post a Comment